Does Prevnar Prevent or Cause Ear Infections?
Many of us have heard that the pneumococcal vaccine, Prevnar, is supposed to provide protection against ear infections -acute otitus media (AOM). The National Academy of Science, the Institute of Medicine, the CDC, AAP, etc. base this assertion on the Prevnar vaccine trials. Dr. Steve Black oversaw the results of the clinical trial in California and stated before an FDA Advisory Committee Meeting held in May 2002 that the control group had 7% more cases of AOM than the group given the Prevnar vaccine. In a clinical trial in Finland, Dr. Terhi Kilpi showed there were 6% more cases of AOM in the control group than the group given the Prevnar vaccine. So, does this mean that Prevnar vaccine protects children from contracting AOM?To answer our question and understand some of the flaws in the vaccine trial, consider this analogy:
In an alcohol trial, 20 subjects were randomized to receive either free whiskey or free vodka.
The next morning 10 whiskey drinkers had 10 hangovers, 100%.
The 10 vodka drinkers had only 9 hangovers, 90%.
Therefore, vodka has an overall protective efficacy of 10 percent against hangovers. Comparison to non-drinkers or moderate drinkers was not made as it is unethical to withhold alcohol from individuals.Before we discuss the flaws in this logic, let us restate the same analogy:
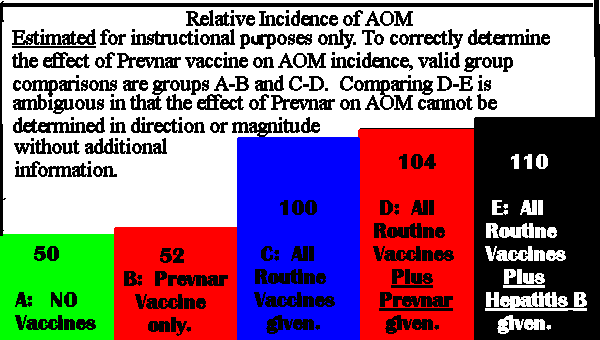
In California, a number of children received all standard vaccines on the CDC recommended schedule. Half the children were also given Prevnar vaccine, and the other half the Investigational meningococcal group C conjugate vaccine (MnCC), the 'control'.
In Finland, a number of children received all standard vaccines on the US CDC recommended schedule. About one third also received Prevnar vaccine, another one third also received Hepatitis B vaccine which formed the 'control' group. The remaining one third also received another investigational vaccine which is discussed very little at the CDC hearing.
The control groups had 6 to 7 percent more AOM than the Prevnar groups.
Therefore, Prevnar 'must be protective' against AOM?
Comparison to an unvaccinated group was not made as it is believed to be 'unethical to leave children unvaccinated.' Comparison was not made between the Prevnar group and a group receiving only routine vaccines because, "it seemed to be the right thing to do to offer something to the control group also, something beneficial."It is obvious that drinking an excess of alcohol causes hangovers, and in comparison a non-drinking group would have NO hangovers. Not equally obvious is that vaccines at least contribute to the causes of AOM. A group of non-vaccinated children would have perhaps HALF the cases of AOM compared to a vaccinated group. The trials do not prove Prevnar prevents any degree of AOM, even the small 6-7 percent quoted. A more likely to be correct interpretation of the available data is that the combination of Prevnar and routine vaccines caused 6-7% less AOM in the trial groups than either 'control' group caused. Using vaccines for 'controls' is flawed methodology and biases results for both safety and effectiveness. The Hepatitis B insert lists Earache and Tinnitus in the reported adverse side effects list.
Many people have observed that unvaccinated children have overall better health than vaccinated children when there is otherwise equal opportunity for attaining optimal health. For more information on the connection between vaccines and increased incidence of ear infections (AOM, etc) see: http://www.vaclib.org/links/ears.htmIn the Finland trial, it was admitted that Prevnar caused a 33% increase in non-vaccine serotype AOM while claiming a 57% decrease of vaccine serotype AOM. ("vaccine efficacy against AOM due to vaccine serotype, 57 percent; there was an increase with non-vaccine serotypes with a negative efficacy of 33 percent;") So does this mean that Prevnar actually prevents more AOM than it causes? First note that this 57% is measured against another vaccine combination, not against an unvaccinated group. Secondly, what does it mean that there was less of a certain serotype AOM? A mucus discharge from the body, whether ear, eye or nose is the bodies effort to detoxify. Serotypes are identified by 'protein signatures'. Since the Prevnar vaccine contains seven of these 'protein signatures' or serotypes then why is there not more of these serotypes present in the AOM ear discharges in the Prevnar group rather than less? I believe the answer lies in Aluminum. Aluminum is a so called adjuvant, but in reality aluminum 'bonds' (adsorbs) to the vaccine proteins and decreases the rate these proteins eliminate from the body while allowing other undesirable protein combinations to be eliminated at a faster rate. A vaccine may change the toxic content that the body is working to eliminate, but this will not make for less illness, only a different diagnosis as to the 'cause'.
It will argued that the reported adverse events of AOM following are too few for the above to be true. However, if there is a large incidence to report ratio, as is commonly verified (10-1 to 100-1 according to the FDA, CDC and a vaccine manufacturers representative.), then it becomes quite feasible that Prevnar does not cause any reduction in the incidence of AOM. Even the Finnish study states that six percent fails, "to reach statistical significance ..."
It must also be noted that vaccine trials are only conducted on healthy children. Logically, both the Prevnar and control groups should have a lower incidence of all diseases than the national average (for vaccinated children). Yet in the Finland trial, it was stated that, "It was suggested that because of the close follow-up during the study that subjects actually sought treatment with ear tubes more often than would ordinarily be the case in Finland, and these rates actually were higher, I think, tenfold higher, nearly tenfold higher than common practice in Finland and also much higher than practice in the Kaiser system." This 10 fold increase in ear-draining tubes is 'explained away' by the fact that during the trial the procedure was provided free. A valid study would have been able to supply data showing what part Prevnar played in either increasing or decreasing the need for ear tubes. Ideally, a vaccine study requires data from many groups, but perhaps four groups from the same time and place would be adequate: a totally unvaccinated group, a group vaccinated only with Prevnar, a group vaccinated with all routine vaccines, and a fourth group vaccinated with Prevnar plus all routine vaccines. Close followup of these children would have provided data for a valid judgement on the effect of Prevnar on AOM. The method used in both the Finland and California trials each contained only one of the required groups, thus NO VALID comparisons could be made.
Entirely too much faith is put on the theory of vaccine protection (antibodies and serotype matches) versus actual measured (clinical) results. Preconceived conclusions currently make it unnecessary for the vaccine industry to conduct valid scientific studies before marketing a vaccine. Even regulatory agencies assume that vaccines give far more benefit than damage even when results are borderline and methodology flawed as was the case in these Prevnar trials. Tests using flawed methodology is the standard in the vaccine industry. Both researchers and regulatory agencies continually assume that vaccines give far more benefit than damage even when methodology is flawed and results are borderline as was the case in both of the cited Prevnar trials. Future vaccine trials and hearings need to have unbiased people with training in modern science to supervise these trials and point out defects in trial design and conclusions. This may not happen soon enough due to the fact that a fair implementing of such oversight would spell the end of vaccines ever reaching the marketplace. However, as more and more people wake up from the vaccine paradigm, changes in the way studies are conducted will be inevitable.
Following is the link to the very long transcript of the FDA hearing on Prevnar/Otitis_Media, LYMErix, etc discussed in this article: http://www.fda.gov/ohrms/dockets/ac/02/transcripts/3854t1.htm
FDA hearing on Prevnar/Otitis_Media, LYMErix, etc
Following is quoted from:
http://www.whale.to/vaccines/baratosy.html
Peter Baratosy, M.B., B.S., Ph.D.An Australian medical practitioner, he uses alternative medicine in his general practice and combined with complementary and orthodox medicines for the maximal benefit for his patients. ...
"I see many children in my practice. Some are immunized and some are not, my own children are not. I see the difference between the immunized and the non-immunized. They're much healthier and have less infections, colds, otitis media and tonsillitis. Dr. Michael Odent has written a letter in the JAMA (1994) where his figures show a five times higher rate of asthma in pertussis immunized children compared to non-immunized children. He is also quoted in the International Vaccination Newsletter (Sept. 1994): "Immunized children have more ear infections and spend more days in hospital."
This, I believe is an indication of immune system suppression due to vaccines. One of the flaws in studies of vaccines is that there are no true placebo groups. The vaccine is tested in one group of immunized children and is compared to another group of immunized children. My advantage is that I have a group of children under my care whose parents have decided not to immunize and I can compare these children with immunized children. The un-immunized are definitely more healthy and so far none have caught any nasty illnesses. The irritability that children experience after immunization is a mild form of encephalitis, which can produce a minimal brain damage. The severity of the initial encephalitis bears no relationship to the eventual damage. This mild damage can cause autism, learning difficulties and hyperactivity. In one study a large proportion of juvenile offenders were discovered to be minimally brain damaged. Minimally brain damaged children were more likely to behave in a violent way."
AOM = Acute Otitis Media
Some Additional Comments
Pneumococcal disease has dropped much faster than vaccine usage has risen. So the vaccine is more than 100% effective. Way to go!
Wow, Could the reduction be due to anything other than the vaccine?
* Were the pre-vaccine stats 'cooked' (inflated) to start with?
* Or post vaccine disease incidence is simply not being fully reported?
* Or factors other than vaccine is causing a lowering of disease incidence?About 55% of children in an small area of Finland were enrolled in the vaccine trial.
No mention was made of the Otitis Media rate in the remaining 45%.
Remember only healthy children were included in the vaccine trial which means the number of expected AOM or any other disease should have been lower than the national average.Even with all the flawed methodology for testing, these two trials for Prevnar are refered too as, "two randomized, well controlled trials". ...
And as to why they used Hepatitis B for the control substance instead of a Placebo, "it seemed to be the right thing to do to offer something to the control group also, something beneficial." SOMETHING BENEFICIAL!
(Didn't anyone tell them that Hepatitis B vaccine causes more adverse events in this age group than Hepatitis B disease causes?)Unfortunately, using a vaccine for the 'control' to another vaccine introduces unknowns, and biases both safety and effectiveness measurements, the exact opposite of what a 'control' is supposed to do. 'Unknowns' include the effect of combining multiple vaccines on the incidence total of AOM. Prevnar plus California routine*, Prevnar plus Finland routine, Hepatitis B plus routine, and meningococcal C plus routine* might all have bias toward different rates of AOM. (*The routine schedule of recommended vaccines changed during the California trial.) All of the vaccines, and more so all of the combinations would likely contribute to causes of AOM. One of the causes would likely be the foreign matter introduced into the body from the vaccines. Another likely contribution to AOM might be treating the side effects of vaccination with antibiotics. Even fever reducing procedures may keep the body more toxic than procedures of allowing the body to fully heal.
Still, none of the discussing group condemned the study as 'flawed methodology'. I think many individuals who have woke to the dangers of vaccines would agree that adequate scientific testing of vaccines is badly needed. Hearings such as this need to have at least one unbiased person who is knowledgeable in modern science, and who answers only to consumers to supervise the trials and point out defects of trial design and conclusions.
-----Following quotes are 'highlights' from the original---------
Source: http://www.fda.gov/ohrms/dockets/ac/02/transcripts/3854t1.htmUNITED STATES OF AMERICA FOOD AND DRUG ADMINISTRATION
CENTER FOR BIOLOGICS EVALUATION AND RESEARCH
VACCINES AND RELATED BIOLOGICAL PRODUCTS ADVISORY COMMITTEE MEETING
This transcript has not been edited or corrected, but appears as received from the commercial transcribing service. Accordingly the Food & Drug Administration makes no representation as to its accuracy.
TUESDAY, MAY 21, 2002
[Following excerps are only a very small part of the original document.][California trials:]
So, in summary, we've observed a dramatic reduction in basic pneumococcal disease in childhood within our population. The magnitude of the reduction in the first year, which was much greater than the vaccine coverage, and the reduction observed in adults suggests herd immunity effect. ...So at the moment, you may be aware that the package insert makes no mention whatsoever about otitis media with regard to Prevnar efficacy, and we are here today to propose that otitis media be included in the package insert and that the indication be that Prevnar is indicated for active immunization of infants and toddlers against invasive disease and otitis media caused by Strep. pneumoniae due to the capsular types included in the vaccine.
And some of the reasons why we believe this is to be important is that there are now two randomized, well controlled trials that you'll hear about which show statistically significant decreases in otitis media outcomes. ...
[Finland trials:]
DR. KILPI: Good morning. I'm going to present the main efficacy results of the Finnish otitis media vaccine trial that evaluated the efficacy of two seven-valent pneumococcal conjugate vaccine for prevention of acute otitis media due to vaccine serotypes in children less than two years of age.And this study was conducted in the Tampere area in Finland, and the clinical phase started in December '95 and ended in March '99, and during this time, we had almost 2,500 children were enrolled in the study. This is approximately 55 percent of the birth cohort in the area.
And all of these children were randomized to receive either one of the two pneumococcal conjugate vaccines used in the study, the PncCRM vaccine labeled, licensed as Prevenar or the PncOMPC vaccine or the control vaccine that was Hepatitis B vaccine in our study. ...
And this is a summary of the main efficacy results, AOM,
vaccine efficacy against AOM due to vaccine serotype, 57 percent;
against culture confirmed pneumococcal AOM, 34 percent;
against pneumococcal AOM confirmed by either culture or PCR, analyzing PCR or both, 20 percent.
These are all statistically significant.
Against any AOM, six percent, and recurrent AOM, 16 percent.
The latter two failed to reach statistical significance in our study. ...And so principally, the indications for tympanostomy tube placement were the same during the vaccine trial and after the trial when the children had returned to the normal life situation, but access to treatment became definitely more difficult when the trial follow-up was over due to the reasons here.
And this makes plain why the incidence of tympanostomy tube placements in the FinOM children during the vaccine trial follow-up was considerably higher than what it is in the children of the same age in Finland in general.
And it also makes plain why this incidence of tympanostomy tube placement dramatically dropped when they returned to a normal life situation. So it appears that milder cases of recurrent AOM and otitis media with effusion were treated with tympanostomy tube placement during the trial and after it, and this makes plain why the effect of the vaccine on the incidence of tube placement was different here from what it was here. ...
And these are the tympanostomy tube placements in the fully evaluated children. During the trial follow-up from two months to two years of age, 20.3 percent of the children in the PncCRM group as compared to 23.8 percent of the children in the control group had tympanostomy tubes place, and the incidence rate of events is here. So the difference between the vaccine group and the control group is 12 percent, and this is not statistically significant. ...
However, when the normal life situation started during the period from two years to four to five years, only 8.2 percent of the children in the PncCRM group as compared to 13 percent of the children in the control group had a tympanostomy tube placement. ...
As shown here, actually the rates of first ear tube placement, number of subjects with events in this table were quite similar and no efficacy estimate was provided. It was suggested that because of the close follow-up during the study that subjects actually sought treatment with ear tubes more often than would ordinarily be the case in Finland, and these rates actually were higher, I think, tenfold higher, nearly tenfold higher than common practice in Finland and also much higher than practice in the Kaiser system. ...
Only in Finland did we have data on vaccine related serotype OM and the related serotypes also showed a significant reduction at 51 percent with reasonably narrow confidence interval, and there was an increase with non-vaccine serotypes with a negative efficacy, as you've heard, of minus 33 percent.
Nevertheless, that increase was counterbalanced by the positive effects within that efficacy for the vaccine against all pneumococci, 33 percent or 34 percent with, again, a reasonably narrow confidence band. ...
DR. KATZ: I guess I wondered why you picked Hepatitis B as the control vaccine. What was the motivation for that?
DR. KILPI: Well, it's not included in the routine program in Finland. It's only recommended for risk groups, and it seemed to be the right thing to do to offer something to the control group also, something beneficial. ...DR. KILPI: We do. We looked at -- what we have in the database is the data on penicillin resistance, but the resistance situation in Finland is very different from that in the U.S. So that almost all of them were susceptible to penicillin.
However, if they were not susceptible they were usually or I think they were exclusively vaccine serotypes. ...In the Finnish study, subjects were randomized equally to one of three vaccines, Prevnar, PnbcOMP manufactured by Merck, and the Hepatitis B vaccine control.
However, only data related to Prevnar were provided in the application and only data related to Prevnar will be discussed today.
The study was double blind, and eligible subjects were in good health as determined by medical history, exam, and clinical judgment.
Of note, infants born prematurely could be enrolled in the study if they were judged to be in good health. ...
[following is some stuff I clipped from the package insert info:
Hep B Recombivax HB (Recombinant)(Merck and Co.)
Special senses
Earache (clinical trials = or < 1% within 5 days)
Tinnitus (post marketing reports)
Engerix-B ®
Hepatitis B Vaccine (Recombinant) (SmithKline Beecham Biologicals)
Additional adverse experiences have been reported with the commercial use of Engerix-B. Those listed below are to serve as alerting information to physicians.
Special senses: Conjunctivitis; keratitis; visual disturbances; vertigo; tinnitus; earache.]From the Prevnar Insert:
Of the 17,066 subjects who received at least one dose of Pneumococcal 7-valent Conjugate Vaccine (Diphtheria CRM197 Protein), Prevnar®, in the efficacy trial, there were 24 hospitalizations (for 29 diagnoses) within 3 days of a dose from October 1995 through April 1998. Diagnoses were as follows: bronchiolitis (5); congenital anomaly (4); elective procedure, UTI (3 each); acute gastroenteritis, asthma, pneumonia (2 each); aspiration, breath holding, influenza, inguinal hernia repair, otitis media, febrile seizure, viral syn-drome, well child/reassurance (1 each). There were 162 visits to the emergency room (for 182 diagnoses) within 3 days of a dose from October 1995 through April 1998. Diagnoses were as follows: febrile illness (20); acute gastroenteritis (19); trauma, URI (16 each); otitis media (15); well child (13); irritable child, viral syndrome (10 each); rash (8); croup, pneumonia (6 each); poisoning/ingestion (5); asthma, bronchi-olitis (4 each); febrile seizure, UTI (3 each); thrush, wheezing, breath holding, choking, conjunctivitis, inguinal hernia repair, pharyngitis (2 each); colic, colitis, congestive heart failure, elective procedure, hives, influenza, ingrown toenail, local swelling, roseola, sepsis (1 each).
For a wonderful in depth article:
http://www.whale.to/v/prevnar2.html
PREVNAR A Critical Review of a New Childhood Vaccine
© Michael Horwin, MA
BBC News: Medical research 'often flawed'
Source: BBC News: http://news.bbc.co.uk/1/hi/health/2352839.stmSaturday, 26 October, 2002, 00:54 GMT 01:54 UK
Medical research 'often flawed'
Medical research is expected to be independent Clinical trials of many new drugs and treatments are flawed and possibly unethical, a study suggests.Experts in the United States have found that many researchers fail to follow international guidelines when they are carrying out studies funded by the pharmaceutical industry.
They also fail to protect their independence and ensure that their findings are published, particularly if the results are unfavourable.
Doctors said the discovery raised serious questions about the integrity of some studies.
They added that it could also stop patients from volunteering to take part in future trials.
Countrywide survey
Doctors at the Duke Clinical Research Institute surveyed 108 medical colleges across the US.
Researchers were asked if they followed guidelines issued by the International Committee of Medical Journal Editors last year.
These guidelines were drawn up by the editors of more than 500 medical journals across the world and are aimed at ensuring studies are robust and of a high standard.
However, the study, which was funded by the institute, revealed that researchers rarely followed the guidelines when they were carrying out research funded by industry.
Just 1% said they had full access to all of the data from the clinical trial. The same proportion said they were able to decide when and where the results of their studies were published.
The survey also showed that many of the contracts between medical colleges and industry were inadequate.
Dr Kevin Schulman, professor of medicine at Duke University Medical Center, said the results were surprising.
"We didn't expect to find full compliance with the guidelines but we were surprised by the extent to which the agreements entered into by medical schools did not protect the independence of investigators in clinical studies and the integrity of their research."
But he added: "This is the first study to look at compliance with the new ICMJE guidelines so we hope it will offer medical schools a road map for how they might improve their agreements with industry sponsors."
Warning
However, Dr Jeremy Sugarman, director of the Center for the Study of Medical Ethics and Humanities at the university, warned that patients could stop volunteering to take part in clinical trials if researchers failed to strengthen their procedures.
"Patients often participate in clinical trials not only for personal benefit, but also because they believe they are contributing to scientific knowledge as a whole.
"If trial data are not made available to others, this may break an implicit promise to research participants that their contributions will be used in such a way."
Dr Michael Wilks, chairman of the British Medical Association's medical ethics committee, said the findings were worrying.
"It is a worry if a drug company is able to find some way of stopping publication of the results of a study which is not favourable to its product."
But he added: "It is different in the UK. Studies are approved by research ethic committees before they can go ahead and I don't think there would ever be a case where they would allow a study to proceed if there was a chance that the drug company could prevent publication of the findings."
The study is published in the New England Journal of Medicine.
Vaccines expert warns studies are useless
Sunday Telegraph 27/10/02
Vaccines expert warns studies are useless
By Lorraine Fraser, Medical Correspondent
(Filed: 27/10/2002)
Most safety studies on childhood vaccines have not been conducted thoroughly enough to tell whether the jabs cause side effects, a leading authority on vaccine research has warned.Dr Thomas Jefferson, who has been funded to investigate vaccine safety by the European Commission, said that the issue was the "Cinderella" of public health research and that Government officials had failed to make it a high priority.
Dr Jefferson is the head of the vaccine division of the Cochrane Collaboration, an organisation of scientists that aims to make accurate information about the effects of treatments available worldwide and promotes high standards in research.
He is also a board member of the European Programme for Improved Vaccine Safety Surveillance, set up by the commission. He said: "There is some good research, but it is overwhelmed by the bad. The public has been let down because the proper studies have not been done."
His outspoken and unprecedented comments will anger public health officials in Britain and elsewhere, who fear that any discussion will undermine parents' confidence in national vaccination programmes. Officials at the Department of Health are already alarmed by the number of parents shunning the triple measles, mumps and rubella jab (MMR) after claims that it is linked with autism and bowel disease.
Although Dr Jefferson emphasised that there was no evidence to suggest that any vaccine now in use was dangerous, he said that there was a "dearth" of sound studies on the risks and benefits.
As a result, the information available on the safety of vaccines that are routinely given to babies and toddlers was "simply inadequate". Dr Jefferson also disclosed plans for a Europe-wide electronic register of children's vaccine exposure that would allow scientists to investigate the risks and benefits of inoculations using data on thousands of participants. Pilot schemes will start soon in Sweden and Finland.
"We need such a system urgently," he said. "Governments are reluctant to accept this but in my view they owe it to future generations to back this idea."
He was especially concerned, he said, because future vaccination programmes were likely to involve giving children "five, six, even seven vaccines all at once". A vaccine designed to protect children against measles, mumps, rubella and chickenpox in one shot is already under development.
"For people like me, it is becoming more and more difficult to tease out what problems may be due to an individual vaccine," said Dr Jefferson. "It is almost becoming impossible to do this. We have to think very carefully about how we will monitor these vaccines.
"We have a responsibility to these children - they are our future. It is no use having a situation where someone suggests a possible harm and everyone runs around frantically trying to find bits of evidence. What is required is good-quality information that has been systematically collated and assessed."
.. Approximately every fifth upper respiratory tract infection is complicated by AOM (5,6).
The incidence of AOM is highest among children under two years of age...
herkules.oulu.fi/isbn9514252314/isbn9514252314.pdf
[following is from pg 16 & 17]
2.1.2. Epidemiology
AOM is closely related to viral respiratory infections and it is one of the most common
diseases in childhood. Approximately every fifth upper respiratory tract
infection is complicated
by AOM (5,6).
The incidence of AOM is highest among children under two years
of age and it gradually diminishes with age, the peak incidence being between the ages of
6 to 12 months (5,7-9).
In a prospective cohort study of 498 children in the area of greater
Boston, the annual incidences of at least one episode of AOMin the first, second and third
year were 62 %, 59 % and 40 %, respectively (9).
In a Finnish cohort study of 2512 children, the cumulative incidence of children who experienced at least one episode of AOM
was 42 % during the first year of life and 71 % by the age of two (8).
An even higher fre-quency
of middle ear disease has been reported in a prospective study from the USA,
where 79 % and 91 % of the 2253 children experienced at least one episode of MEE
before the ages of one and two, respectively (10).
Small differences in
these studies may
be partly due to variable numbers of drop outs, differences in diagnostic
criteria or variation
in microbiological aetiology.
The incidence rates of recurrent AOM in various studies are summarized in table 1.
Table 1. Incidence of recurrent AOM episodes in different studies. AOM, acute otitis media; y,years; mo,months; n, number of children in cohort Author Criteria n % Sipilä et al. 1987 (7) ³3 AOM before age of 18 mo 1642 30 Teele et al. 1989 (9) ³6 AOM before age of 3 y 498 16 Harsten et al. 1989 (11) ³6 AOM in 12 mo period before age of 36 mo 113 12 Ingvarsson et al. 1990 (12) ³4 AOM before age of 4 y 2978 18 Duncan et al. 1993 (13) ³3 AOM in 6 mo or 4 AOM before age of 12 mo 1220 17 Alho 1997 (14) ³3 AOM in 6 mo period in children aged under 24 mo 2411 15The incidence of AOM appers to have increased in the past few decades.
Schappert found about 2.5 times more clinic visits with a principal diagnosis of OM in the United States during 1990 than during 1975 (15). The incidence of recurrent OM among pre-school children has also increased from 18.7 % in 1981 to 26 % in 1988 in the United States (16).
The same tendency has been found in Finland, where the total number AOM episodes was estimated to be 200 000 in 1982 and 500 000 in 1997 (17,18).
[In a reference to the Finland trial for Prevnar]
[PDF] Medicine Digest 9 March 2001 Number 196 North West Medicines
www.ukmi.nhs.uk/NewMaterial/html/docs/09030102.pdf
1662 children attending child health clinics in Finland were randomised to receive the pneumococcal conjugate vaccine or the control (hepatitis B) vaccine. The conjugate vaccine contained a number of pneumococcal serotypes. The children received either the study or control vaccine at 2,4,6, and 12 months of age with follow-up at 13,18 and 24 months of age. The clinical diagnosis of AOM was based on predefined criteria, and bacterial diagnosis was based on a culture of middle-ear fluid. A total of 2596 episodes of clinical AOM were diagnosed among the study and control vaccine groups during the follow-up period between 6.5 and 24 months. Pneumococcal vaccine was associated with a reduction of 6% (95% CI, -4 to 16% [the negative number indicates a possible increase in the number of episodes]) in the number of episodes of AOM from any cause.
http://www.whale.to/m/pneumococcal.html
"Pneumococcal Vaccine and Otitis Media" by Dr. Erdem Cantekin
Cantekin discussed the new Prevnar vaccine for pneumococcal, as endorsed by the American Academy of Pediatrics. "The alleged benefits for this new vaccine are greatly exaggerated and the risks are significant,"
http://www.whale.to/v/prevnar.htm
Prevnar (page of links and quotes)
"The Prevnar pre-licensure clinical trials, which Wyeth Lederle paid Kaiser Permanente to conduct, compared two experimental vaccines against each other. To compound this basic methodological flaw, Kaiser and Wyeth Lederle, allowed most of the children in the trial to be given the more reactive DPT vaccine rather than use the safer, less reactive DTaP vaccine. This placed the children in that five-year experiment in greater danger and allowed the drug company to write off the seizures that occurred as being caused by DPT and not Prevnar, when in fact, they didn't know. Even so, the groups of children who got Prevnar suffered more seizures, higher fevers, more irritability and other reactions than did the children who got the other experimental vaccine. It was a no-brainer as far as I was concerned: Kaiser and Wyeth Lederle had proved nothing about Prevnar vaccine safety."--Barbara Loe Fisher
List of Prevnar links in TABLE OF CONTENTS
Recently a large pneumococcal vaccine trial started in Finland with the aim of recruiting more than 90 000 children aged 6 weeks to 18 months. This trial gives Hepatitis B or Hepatitis A vaccines to the control group instead of a placebo.
Read rest of the story at::
Press release 16.3.2009: Vaccine trial violates ethical code
Finnish NGO: GlaxoSmithKline pneumococcal vaccine trial in Finland violates ethical code
http://www.rokotusinfo.fi/en/release_090316_html
The vaccine in the trial is known by the names Synflorix, PhiD-CV, Streptorix, GSK1024850A, GSK1024850.